Gene Therapy
viral vector production
Overview

Sub-Applications
Cell line generation with
>99.99% probability of clonality
Generate clonally derived cell lines and decrease batch-to-batch variations for an improved viral vector manufacturing process.
Improve suspension cell culture and clonal expansion
Optimize cell culture and establish a high-throughput clonal expansion workflow to effectively select cell lines.
Reduce timelines
for clonal expansion
Save up to 13 weeks on your cell line development workflows and increase throughput from single-cell cloning to clonal cell culture and upscaling.
Research Workflows
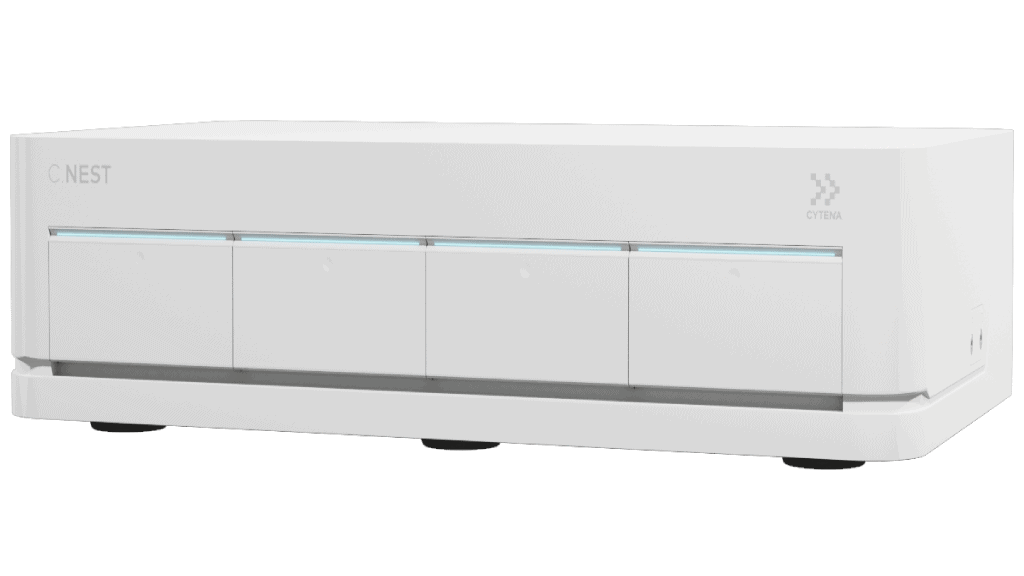
Streamline cell line development from the early stages after transfection. With the UP.SIGHT, quickly isolate single cells and track colony growth for an initial impression of cell viability. Add our microplate agitation culture system, the C.NEST, to increase cell culture quality and cultivate clonal cell lines in a completely controlled and monitored environment.
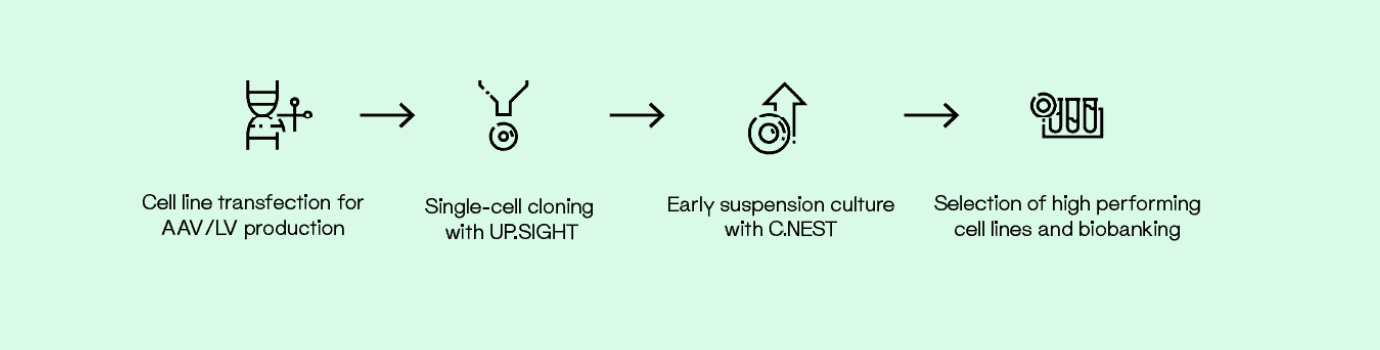
For ex vivo approaches in which cells are genetically modified during culture, the C.NEST is a high-throughput microbioreactor in which 24- or 96-well culture plates are continuously monitored to optimize and upscale clonal expansion. It offers continuous mixing for suspension cells in 96-well and 24-well plates by providing a higher diffusion rate of oxygen, which creates a more homogeneous
culture environment.