- PRODUCTS
-
UP.SIGHT™ 2nd GenNEW
Optimized For Proof Of Monoclonality, Colony Tracking, Confluency, & Titer Measurement -
F.SIGHT™ 2.0
Optimized For Rapid Dispensing of Fluorescent Cells -
C.SIGHT™ 2.0
Optimized for Powerful Dispensing of Unlabeled Cells -
B.SIGHT™
Optimized For Rapid Microbial Single-Cell Isolation and Cultivation -
F.SIGHT™ OMICS
Optimized For Single-Cell-Omics -
F.SIGHT™
Optimized For Affordability And Flexibility -
C.SIGHT™
Optimized for Affordable Cell Line Development -
Compare Products
Decide which one is right for you - Help Me Choose
-
UP.SIGHT™ 2nd GenNEW
- APPLICATIONS
- RESOURCES HUB
- COMPANY
- SHOP
Cell line development
Generating clonally derived cell lines for the development
and production of biotherapeutics.
and production of biotherapeutics.
Overview
With current advances in technology and biopharmaceutical research, cell line development (CLD) has become a prominent process in many application areas. One of these areas would be the development of biotherapeutics, including a variety of monoclonal antibodies, vaccines and biologics. The development of such new medical treatments has seen a sharp increase in demand for better quality and safety standards. In order to meet the increasing demand for these types of products, researchers rely on different mammalian expression-based systems, with CHO cells being the most popular system for large-scale
industrial culturing.
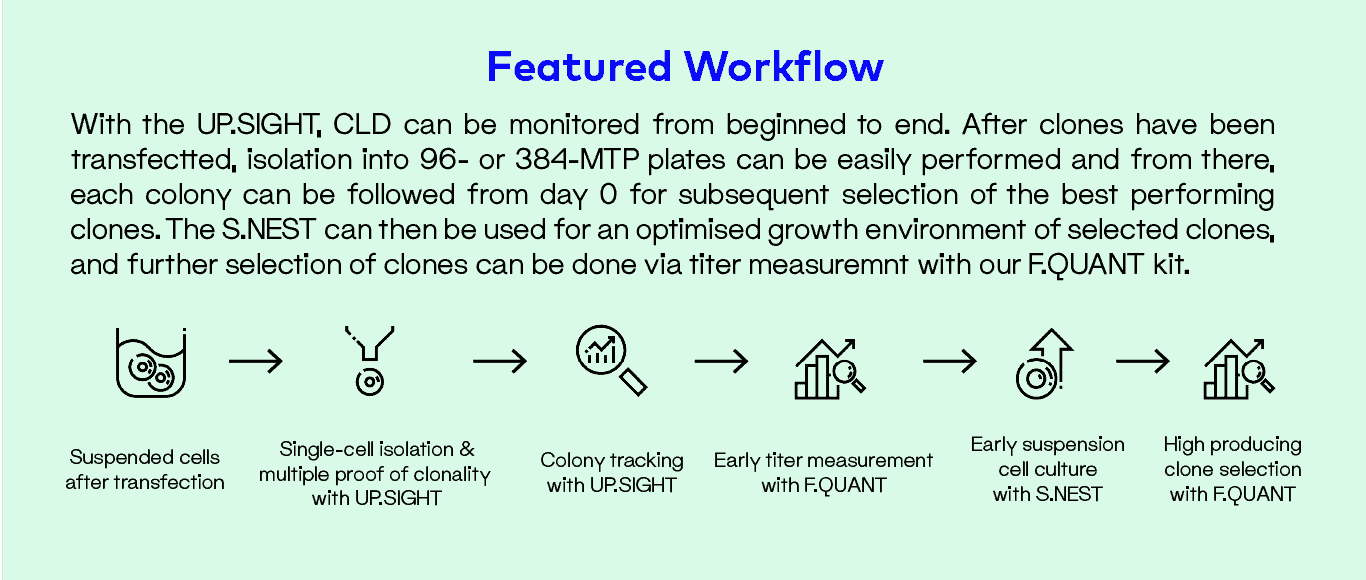
Throughout the use of cell-based assays, researchers have been screening different clones in order to increase productivity of biopharmaceuticals. The manual screening methods traditionally used for CLD are time consuming and labor intensive. As such, there is a great demand for high-throughput, automated solutions for better processes that follow GMP and legal safety standards while also drastically reducing the production timeline. The proposed workflow using our UP.SIGHT, S.NEST and F.QUANT helps to streamline and optimize your research to generate high-producing cell lines with a probability of clonal derivation of >99.99%.
Sub-Applications

Monoclonal
antibody production
Accelerate your CLD workflows using automated and high-throughput single-cell isolation technology and establish high-producing clones with >99.9 % probability of clonal derivation.
Viral vector production
for gene and cell therapy
Generate clonally derived cell lines producing high titers of viral particles for efficient gene editing in various target cell types.
iPSC cell line engineering
for cell therapies
Streamline time-consuming steps of your iPSC workflows and improve quality control by ensuring the clonal derivation of iPSC cell lines, which ultimately improves cellular therapies in regenerative medicine.
Research Workflows
Single-cell Cloning and Proof of Clonality
Following transfection, single cells need to be isolated from transfected pools. Traditionally, this crucial step has been performed by limiting dilution. Based on early regulatory guidelines released by the U.S. Food and Drug Administration (FDA) and others, the production cell line of recombinant products is to be derived from a single cell in order to minimize population heterogeneity as well as facilitate isolation and subsequent selection of high-producing clones.
Single cell dispensing
Our single-cell dispensing technology enables deterministic single-cell isolation with a documented proof of clonality >99.99% and provides efficient single-cell seeding combined with excellent cell viability and minimal risk of cross contamination.
Colony tracking
Colony growth can be tracked via confluency measurement using the plate imaging capacities of the UP.SIGHT and our intuitive analysis software, C.STUDIO.
Titer measurement
High-producing clones can be selected using CYTENA’s F.QUANT titer assays to determine monoclonal antibody or Fab fragment concentrations from the culture supernatant in a fast and easy way. F.QUANT can be used as a standalone titer measurement on third party instruments or can be used within our fully automated workstation, the C.STATION.
Early Suspension Culture
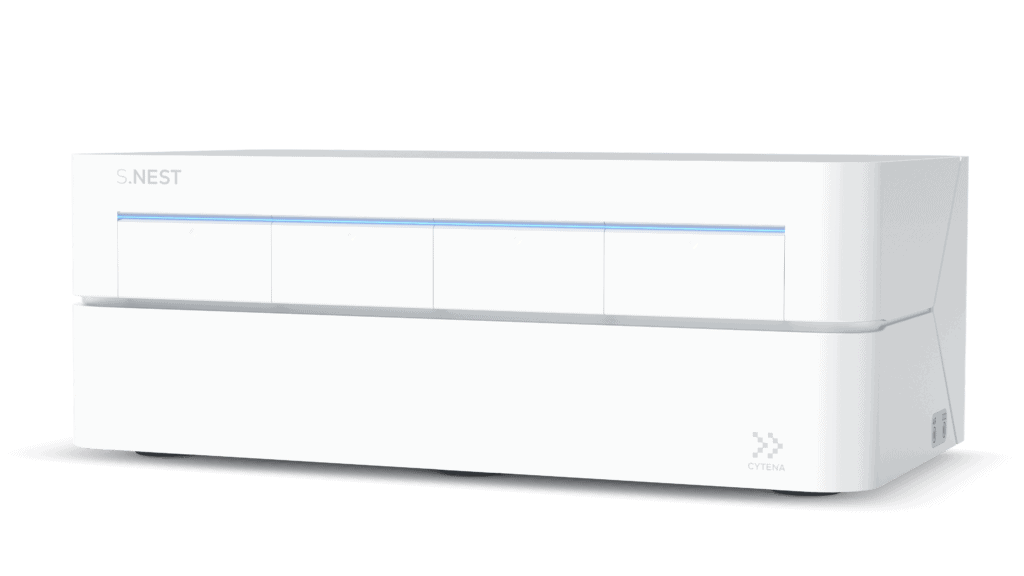
After single-cell cloning, clones exceeding a certain confluency threshold are selected for suspension culture in order to expand cell numbers. Traditional workflows for cell culture happen under static conditions, taking up several weeks of work before viable clones are transferred to shaken deep well or 24-well plates. After additional cultivation, selected clones are then transferred to larger volumes and finally to shake flasks or mini bioreactors. Hence, during the first weeks after cloning, cells are cultured under static conditions, which do not match the culture environment the cells experience in later stages of the process and during production. Our innovative microbioreactor platform, the S.NEST™, was developed to enable suspension culture early in the CLD process. With the S.NEST platform, cells are kept in a more suitable environment thanks to the circulating flow of medium, thus maintaining homogenous levels of nutrients and dissolved oxygen.
Fully automated CLD
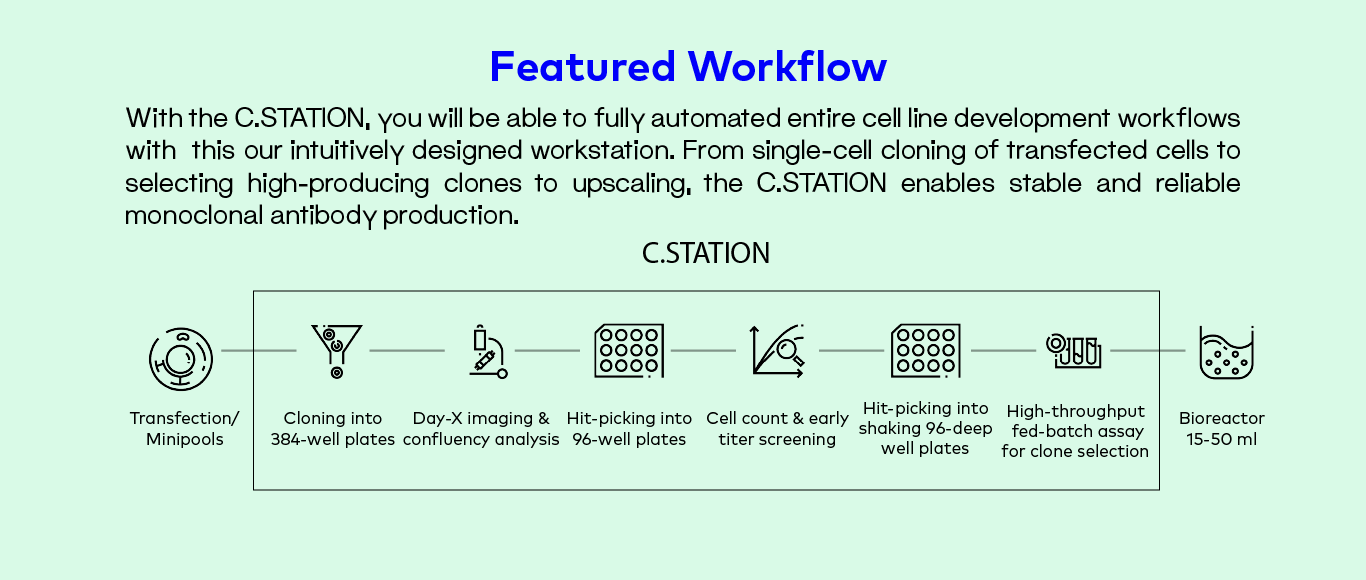
Fully automated CLD with the C.STATION
The next step towards reduced timelines without compromising quality is the integration of all the steps so far mentioned into a single, automated workstation. The C.STATION is CYTENA’s automated workstation covering the full CLD workflow from single-cell cloning to selection and upscaling of best-producing clones. The automation and integration of these stages not only reduces hands-on time, but also brings more reproducibility and trackability to the full CLD process.
One of the main advantages of upgrading to this automated solution is the higher and more diverse experimental capabilities that can be reached, which in turn allows for more sophisticated early screens. With the C.STATION, this means:
- Up to 32 384-well plates during single-cell cloning
- Option to measure titer levels with F.QUANT early on, even before colony picking
- Ability to perform cell count in high-throughput mode with minimal sample amount
- Intuitive software that generates clone pick lists based on internal and external metrics
- Full trackability with clonal derivation assurance, materials and reagent documentation
- Possibility to mirror, split and scale up cultures without manual intervention
- Up to 14 deep-well plates on shaken cultures
- Fed-batch assay on 96 deep well plate format allows productivity evaluation and method optimization (media, feeds) early on
The C.STATION is optimized for mABs production but can also be tailored for viral vector production in gene therapy applications.
Related Application Notes
No Posts Found.
Featured Resources
Previous
Next