Cell Therapy & Stem Cells
Accelerating and automating single-cell cloning of eukaryotic cells
to improve the development of cancer treatments and
advance regenerative medicine.
to improve the development of cancer treatments and
advance regenerative medicine.
Overview
Cell-based therapies are prominent in modern research and are progressing faster than ever, pushing medicine away from relying on palliative care to a focus on developing more effective treatments. For over 30 years, stem cell therapies have been widely used to treat leukemia and lymphoma patients by transplanting hematopoietic stem cells from healthy donors. Nonetheless, these therapies present obstacles similar to those in any other transplant procedure, including a lack of donor matches and a risk of rejection.
These challenges were recently tackled with new autologous cell therapies, including chimeric antigen receptor (CAR) T-cell therapy, which has been approved by the U.S. Food and Drug Administration (FDA) for acute lymphoblastic leukemia since 2017. To treat patients with these primary cell therapies it is highly important that the viral particles used to genetically modify the cells are manufactured under standardized conditions and derive from a clonal cell line.
Even so, standardization of cell manufacturing processes is similarly critical to generate safe cell therapies against neurodegenerative diseases since clinical outcomes and effectiveness of treatment for these diseases are difficult to measure.
CYTENA has developed several instruments which enable the standardization of generating cell products of clonal origin by making the identification, isolation and imaging of single cells more reliable and efficient. A workflow using one of our single-cell dispensing platforms and the CELLCYTE X can optimize this process and ensure that only the best clones are selected, increasing the odds of safer and more effective cell therapies.
Sub-Applications
Viral vector production for cellular therapies
Generate clonally derived cell lines producing high titers of viral particles for efficient genetic engineering of therapeutic cell pools, including CAR T cells or T-cell Receptor (TCR) transgenic cells for cancer treatment.
iPSC cell line engineering
Streamline time-consuming steps in your iPSC workflows and improve quality control by ensuring the clonal derivation of iPSC cell lines to improve cellular therapies in regenerative medicine.
Research Workflows
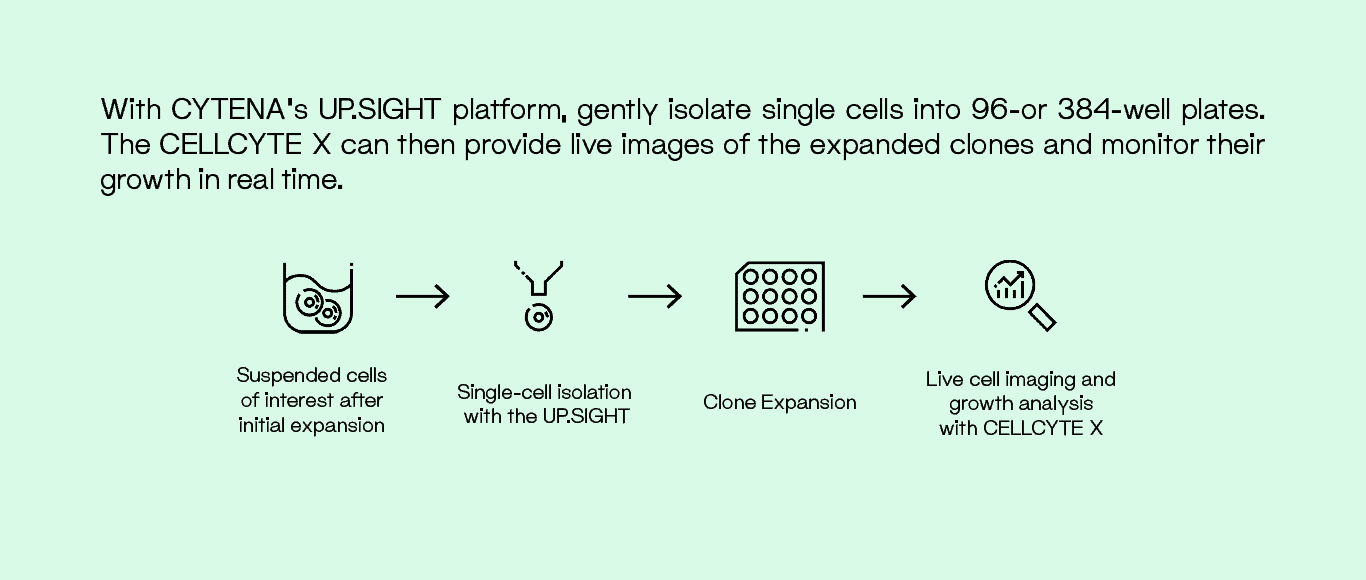
Isolate Cells and Monitor Growth
A combination of the UP.SIGHT single-cell dispensing platform and the CELLCYTE X, live cell imaging platform, can be used to isolate cells of interest and monitor growth seamlessly and efficiently. Gentle dispensing technology minimizes cell stress during isolation. After initial clonal expansion, the CELLCYTE X tracks and monitors cell growth inside the incubator, minimizing stress from manual handling.
Related Application Notes
No Posts Found.
Featured Products
Featured Resources
Previous
Next