- PRODUCTS
-
UP.SIGHT™ 2nd GenNEW
Optimized For Proof Of Monoclonality, Colony Tracking, Confluency, & Titer Measurement -
F.SIGHT™ 2.0
Optimized For Rapid Dispensing of Fluorescent Cells -
C.SIGHT™ 2.0
Optimized for Powerful Dispensing of Unlabeled Cells -
B.SIGHT™
Optimized For Rapid Microbial Single-Cell Isolation and Cultivation -
F.SIGHT™ OMICS
Optimized For Single-Cell-Omics -
F.SIGHT™
Optimized For Affordability And Flexibility -
C.SIGHT™
Optimized for Affordable Cell Line Development -
Compare Products
Decide which one is right for you - Help Me Choose
-
UP.SIGHT™ 2nd GenNEW
- APPLICATIONS
- RESOURCES HUB
- COMPANY
- SHOP
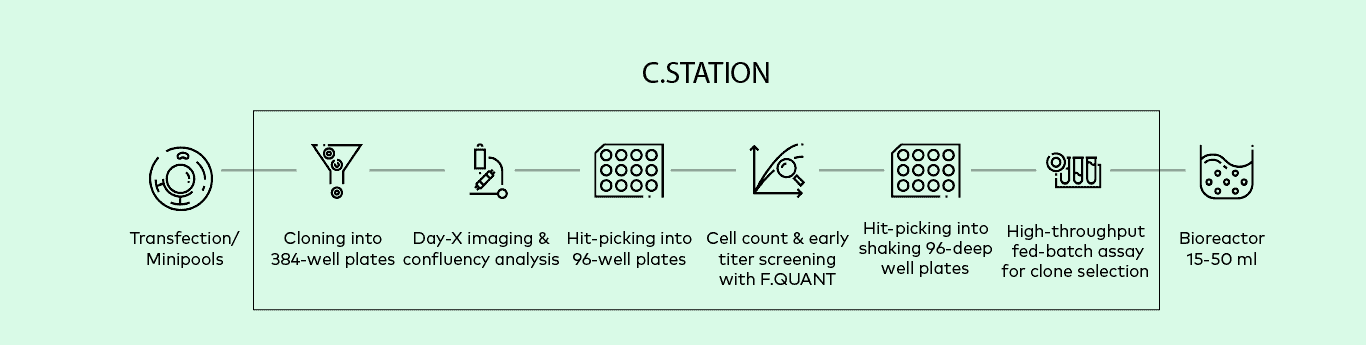
C.STATION™
Optimize and streamline entire cell line development (CLD) workflows with this automated, intuitively designed workstation. From single-cell cloning of transfected cells to selecting high-producing clones to upscaling, the all-new C.STATION fully automates stable CLD for monoclonal antibody production.
C.STATION™
Optimize and streamline entire cell line development (CLD) workflows with this automated, intuitively designed workstation. From single-cell cloning of transfected cells to selecting high-producing clones to upscaling, the all-new C.STATION fully automates stable CLD for monoclonal antibody production.
Join 1,000+ Biopharmaceutical Companies and Academic Institutions



















Key Features & Benefits
Find out how our product can enrich your research

Preconfigured and validated protocols
Optimized and validated protocols help generate stable cell lines and make laboratory automation best practices more accessible.

Flexibility and upscaling
The C.STATION gives you the flexibility to screen hundreds of clones for high titers. Easily upscale CLD by processing multiple runs at once for in-house or contract development manufacturing (CDM).

Proven technology
The C.STATION automates CLD workflows by integrating our proven technologies in single-cell dispensing, assurance of clonality, plate imaging, colony tracking and titer assessment all in a self-contained workstation.

Sterile workflow
HEPA filters within the C.STATION create a sterile environment throughout the entire workflow.
Shorter timelines and better outcomes
The C.STATION is ready to use straight from the box and full automation saves you time normally spent in the planning phase. Our proven technology within the workstation also reduces the risk of failures in the development process.

Single point of contact
CYTENA is your single point of contact for the entire workstation.
Product Details
Discover what you can do
What is inside?
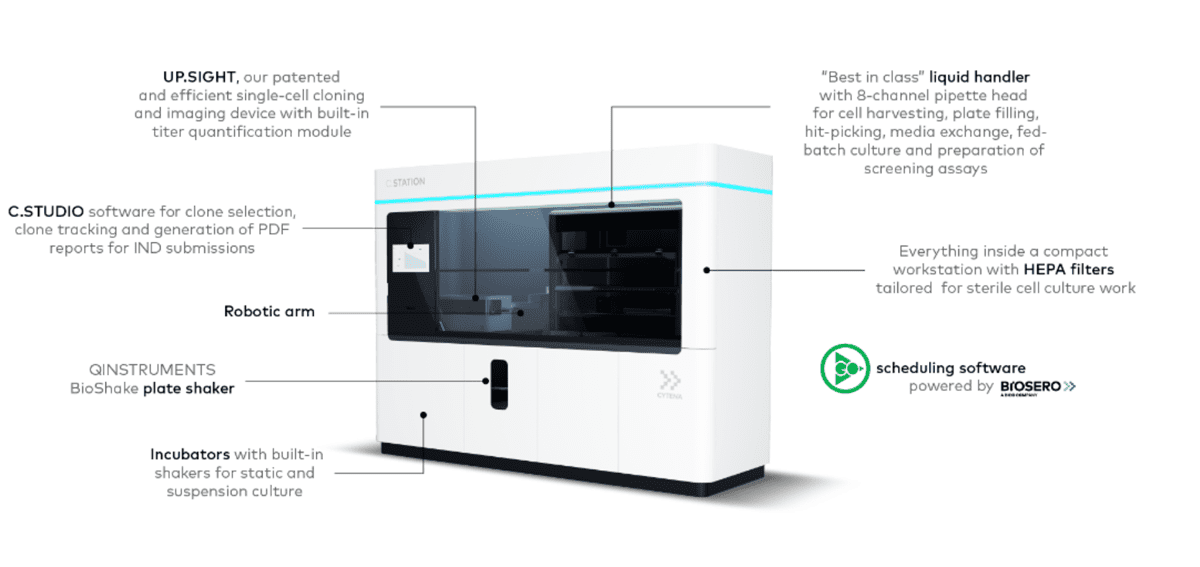
Single-cell cloning to
meet regulatory requirements
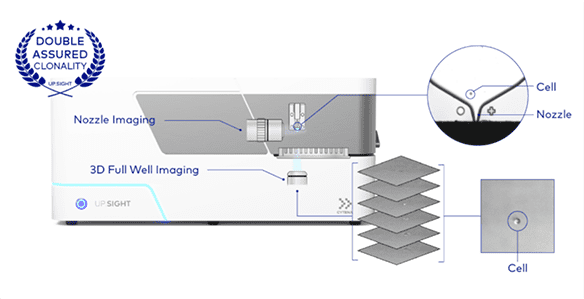
The C.STATION integrates CYTENA’s flagship instrument, the UP.SIGHT™, to enable a probability of clonality >99.99% and fulfill required regulatory expectations. Double assurance of clonality is achieved through nozzle images and 3D Full Well Imaging, a new and innovative method that images the full volume of each well from the point of dispensing using the integrated imager. First, images are taken at the dispensing nozzle to ensure only one cell is placed in each well and nonclonal droplets are discarded. Then, the single cells are confirmed once more with 3D Full Well Imaging.
Tracking colony
growth over time
growth over time
Confluency, cell count and viability of clonal cells are effortlessly monitored by the UP.SIGHT. The C.STUDIO software lets you track growing colonies over the course of an experiment while the instrument enables you to rewind and replay images, providing a full picture of the CLD workflow. With the confluency tool, determine the right time point to pick clones for a larger volume plate format.
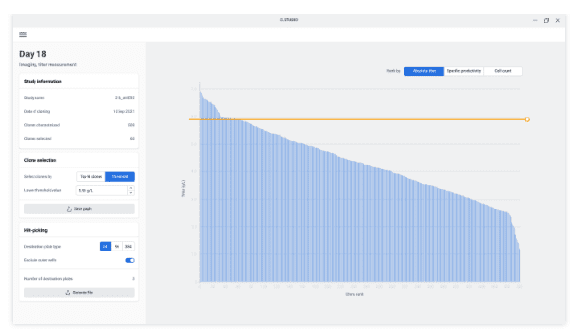
Comprehensive mAb titer monitoring for high-producing clone identification
The C.STATION comes with built-in titer quantification based on a plate-based assay using the F.QUANT. This makes it possible to screen hundreds of clones without user interaction. With the C.STUDIO, clone selection based on relative productivity is straight forward and guarantees data traceability.
Product
Applications
Accelerate your research

Automated cell line development
Monoclonal antibody development

Monoclonal cell culture
Gene therapy
Cell therapy
Featured Workflow
With the C.STATION, you will be able to fully automated entire cell line development workflows
with this our intuitively designed workstation. From single-cell cloning of transfected cells to
selecting high-producing clones to upscaling, the C.STATION enables stable and reliable monoclonal antibody production.
with this our intuitively designed workstation. From single-cell cloning of transfected cells to
selecting high-producing clones to upscaling, the C.STATION enables stable and reliable monoclonal antibody production.
Download Application Notes
For scientists by scientists, these notes highlight novel ways to optimize
your research with our products and solutions
your research with our products and solutions
No Posts Found.
Product Datasheet
| C.STATION Suspension BSL1 | C.STATION Suspension BSL2 | C.STATION Adherent BSL2 | |
| Biosafety Standard | Class I according to NSF/ANSI 49 A2 respect. BS EN 12469:2000 (product protection) HEPA H14 filtered intake | Class II according to NSF/ANSI 49 A2 respect. BS EN 12469:2000 (product and user protection) HEPA H14 filtered intake and exhaust 70/30 recirculation principle with 30% exhausted air | Class II according to NSF/ANSI 49 A2 respect. BS EN 12469:2000 (product and user protection) HEPA H14 filtered intake and exhaust 70/30 recirculation principle with 30% exhausted air |
| Plate Compatibility | ANSI SLAS1-2004 (R2012) without FB module: up to 6-Well with FB module: up to deep-well | ANSI SLAS1-2004 (R2012) without FB module: up to 6-Well with FB module: up to deep-well | ANSI SLAS1-2004 (R2012) up to 6-Well |
| Fed-Batch Option | yes | yes | no |
| Plate Capacity | without FB module: up to 60 plates with FB module: up to 44 plates | without FB module: up to 60 plates with FB module: up to 44 plates | up to 60 plates |
| Cell Sorting | Cell Morphology and Fluorescence on CYTENA UP.SIGHT | Cell Morphology and Fluorescence on CYTENA UP.SIGHT | Cell Morphology and Fluorescence on CYTENA UP.SIGHT |
| Plate Washer | no | no | CYTENA C.WASH |
| Liquid Handling | Hamilton STARlet M | Hamilton STARlet M | Hamilton STARlet M with optional 96-Well head (MPH) |
| Liquid Handling Pipette Tips | 50/300/1000 µL filtered or unfiltered, capacitive tips *unfiltered 50/300 µL tips stackable for 4x capacity on deck | 50/300/1000 µL filtered or unfiltered, capacitive tips *unfiltered 50/300 µL tips stackable for 4x capacity on deck | 50/300/1000 µL filtered or unfiltered, capacitive tips *unfiltered 50/300 µL tips stackable for 4x capacity on deck |
| Liquid Handling Specifications | Minimum/maximum aspirate and dispense volume 50 µL tip 300 µL tip 1000 µL tip | 1-1000 µL depending on tip type @1 µL: 4.0% precision, 5.0% trueness @50 µL: 0.75% precision, 2.0% trueness @200 µL: 0.75% precision, 1.0% trueness @1000 µL: 0.75% precision, 1.0% trueness | 1-1000 µL depending on tip type @1 µL: 4.0% precision, 5.0% trueness @50 µL: 0.75% precision, 2.0% trueness @200 µL: 0.75% precision, 1.0% trueness @1000 µL: 0.75% precision, 1.0% trueness |
| Liquid Handling Troughput | Fill one 96-well microtiter plate with 100 µL samples (new tips for each sample): 320 s Aliquot 100 µL to each well of a 96-well plate, liquid level detection on aspirate: 35 s | Fill one 96-well microtiter plate with 100 µL samples (new tips for each sample): 320 s Aliquot 100 µL to each well of a 96-well plate, liquid level detection on aspirate: 35 s | Fill one 96-well microtiter plate with 100 µL samples (new tips for each sample): 320 s Aliquot 100 µL to each well of a 96-well plate, liquid level detection on aspirate: 35 s |
| Incubation Temperature | RT+5 °C to 37 °C optional cooling: 4°C to 50 °C | RT+5 °C to 37 °C optional cooling: 4°C to 50 °C | RT+5 °C to 37 °C optional cooling: 4°C to 50 °C |
| Incubation CO₂ | 0-20 Vol% CO₂ | 0-20 Vol% CO₂ | 0-20 Vol% CO₂ |
| Incubation Humidity | without FB module: < 95% with FB module: < 80% | without FB module: < 95% with FB module: < 80% | < 95% |
| Plate Shaking for Assays | 200-3000 rpm, constant 2 mm diameter | 200-3000 rpm, constant 2 mm diameter | 200-3000 rpm, constant 2 mm diameter |
| Software | Green Button Go Scheduler, C.STUDIO (Analysis) on Windows 11 | Green Button Go Scheduler, C.STUDIO (Analysis) on Windows 11 | Green Button Go Scheduler, C.STUDIO (Analysis) on Windows 11 |
| Computation | Custom Rack PC | Custom Rack PC | Custom Rack PC |
| Dimensions (W x D x H) | 3500 x 1200 x 2400 mm 137.80 x 47.25 x 94.5 in | 4200x1550x2440 mm 165.50 x 61.10 x 96.10 in | 4200x1550x2440 mm 165.50 x 61.10 x 96.10 in |
| Footprint Service Mode (W x D) | 3500 x 2540 mm 137.80 x 100.00 in | 4650 x 2240 mm 183.10 x 88.25 in | 4650 x 2240 mm 183.10 x 88.25 in |
| Weight | 1500 kg 3310 lbs | 2000 kg 4410 lbs | 2000 kg 4410 lbs |
| Area load | < 400 kg/m² < 85 lbs/ft² | < 500 kg/m² < 105 lbs/ft² | < 500 kg/m² < 105 lbs/ft² |
| Power Supply | 400 VAC, 32 A via IEC 60309 6h (3L+N+PE) option: 3x individually fused line: 230 VAC, 16 A | 400 VAC, 32 A via IEC 60309 6h (3L+N+PE) option: 3x individually fused line: 230 VAC, 16 A | 400 VAC, 32 A via IEC 60309 6h (3L+N+PE) option: 3x individually fused line: 230 VAC, 16 A |
| Ambient Conditions | +15 °C to + 25 °C non-condensing air (30% – 80% rH) | +15 °C to + 25 °C non-condensing air (30% – 80% rH) | +15 °C to + 25 °C non-condensing air (30% – 80% rH) |
Thank you for your interest in our product. We understand that every business has unique needs, and our pricing plans are tailored to meet those needs. For a customized quote and to learn more about our pricing options, please fill out the quote request form. We would be happy to help you find the best plan for your needs and budget and answer any questions you may have. Thank you!
Featured Resources
Donec scelerisque scelerisque neque, non sagittis ligula malesuada at.