GENETICALLY ENGINEERED SINGLE-CELL CLONES
Characterizing CRISPR/Cas9-engineered cells is a major priority for pharmaceutical companies, as well as for novel cellular therapeutic applications and regenerative medical uses. For this reason, the team at the Department of Microsystems Engineering at the University of Freiburg, under Dr. Stefan Zimmermann’s leadership, developed a new workflow focused on improving the characterization of CRISPR/Cas9-engineered cells.
That innovative high-throughput, cost-effective and streamlined single-cell workflow combines the F.SIGHT™’s fluorescent single-cell dispensing technology, a genomic editing screen called surveyor assay, RT-PCR mRNA assessing altered gene expression, and a versatile protein detection tool called emulsion coupling.
Lorem
ipsum dolor
- Lorem Ipsum is simply dummy text of the
- Lorem Ipsum is simply dummy text of the
- Lorem Ipsum is simply dummy text of the
- Lorem Ipsum is simply dummy text of the
GENETICALLY ENGINEERED SINGLE-CELL CLONES
GENETICALLY ENGINEERED SINGLE-CELL CLONES
Characterizing CRISPR/Cas9-engineered cells is a major priority for pharmaceutical companies, as well as for novel cellular therapeutic applications and regenerative medical uses. For this reason, the team at the Department of Microsystems Engineering at the University of Freiburg, under Dr. Stefan Zimmermann’s leadership, developed a new workflow focused on improving the characterization of CRISPR/Cas9-engineered cells.
That innovative high-throughput, cost-effective and streamlined single-cell workflow combines the F.SIGHT™’s fluorescent single-cell dispensing technology, a genomic editing screen called surveyor assay, RT-PCR mRNA assessing altered gene expression, and a versatile protein detection tool called emulsion coupling.
Characterizing CRISPR/Cas9-engineered cells is a major priority for pharmaceutical companies, as well as for novel cellular therapeutic applications and regenerative medical uses. For this reason, the team at the Department of Microsystems Engineering at the University of Freiburg, under Dr. Stefan Zimmermann’s leadership, developed a new workflow focused on improving the characterization of CRISPR/Cas9-engineered cells.
That innovative high-throughput, cost-effective and streamlined single-cell workflow combines the F.SIGHT™’s fluorescent single-cell dispensing technology, a genomic editing screen called surveyor assay, RT-PCR mRNA assessing altered gene expression, and a versatile protein detection tool called emulsion coupling.
IMPROVING IDENTIFICATION AND CHARACTERIZATION CRISPR/CAS9 KNOCK-OUT TECHNOLOGY
The F.SIGHT is particularly suitable for cloning of engineered cells because, in addition to preserving their viability, it provides direct evidence of single-cell clonality and has both native and fluorescent sorting capabilities. In addition, the F.SIGHT can be coupled with single-cell analytical pipelines and requires far lower sample volumes to process than FACS, allowing researchers to isolate cells from limited samples. Finally, even when the fluorescence of the transfected cells is very limited, researchers can still identify the target population with the F.SIGHT, because it is able to read low signals.
Another innovative assay used in the workflow developed by Dr. Zimmermann’s team is emulsion coupling based on a digital method similar to droplet digital PCR (ddPCR), which detects the differences in partitioning of two antibodies labeled with target oligonucleotides in the presence of target proteins compared to their free and unbound distribution in ddPCR emulsion. Emulsion coupling is homogeneous, molecularly sensitive and quantitative. Plus, because of this assay’s two-antibody principle, it is highly specific and can be read by sequencing (e.g., next-generation sequencing) in a highly parallel manner.
A TECHNOLOGICAL SOLUTION DESIGNED TO OVERCOME THE CURRENT LIMITATION
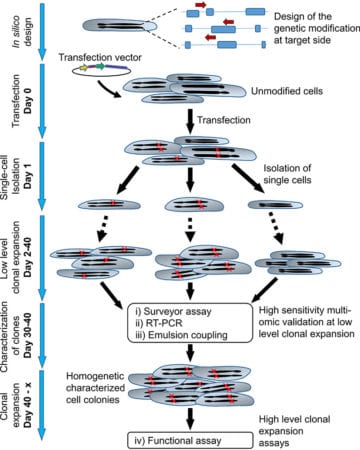
- Genome-wide changes were detected using a surveyor assay
- Target gene transcript levels were monitored by RT-PCR
- Target protein expression by clones was measured by Emulsion coupling
References:
Gross T, JeneyC, Halm D, et al. Characterization of CRISPR/Cas9 RANKL knockout mesenchymal stem cell clones based on single-cell printing technology and Emulsion Coupling assay as a low-cellularity workflow for single-cell cloning. PLoS ONE. 2020; 16(3): e0238330. DOI:1371/journal.pone.0238330.
EXPLORE THE BENEFITS OF F.SIGHT™
FEATURED WORKFLOW
Transfection

Transfection

Transfection
IMPROVING IDENTIFICATION AND CHARACTERIZATION CRISPR/CAS9 KNOCK-OUT TECHNOLOGY
As part of this experimental proof of concept, immortalized mesenchymal stem cells (MSCs) were genetically modified to enhance their ability to form bone for potential regenerative medical applications. The Tumor Necrosis Factor Superfamily Member 11 (TNFSF11), which encodes the nuclear factor kappa B ligand activating receptor (RANKL), was deleted.
RANKL has previously been shown to play a crucial role in bone homeostasis by orchestrating the balance between osteoblasts that generate bone and osteoclasts that degrade it, primarily because RANKL is expressed in bone tissue by mesenchymal stem cells, osteoblasts and T cells. In the presence of RANKL, the nuclear factor kappa B activating receptor (RANK) is activated, which stimulates pre-osteoclasts to differentiate into osteoclasts that in turn degrade bone, and for bone formation, MSCs differentiate into osteoblasts and deposit calcified structures.
By knocking out TNFSF11, genetically modified MSCs and their progenitors can no longer recruit osteoclasts. Therefore, bone formation on the sidelines of their implantation in a regenerative medical application could potentially be improved.
One of the technologies that most aided the development of this workflow is CYTENA’s single-cell isolation technology, which is a gentle, low-cost and highly controlled technology applicable for a wide range of specific cell cloning applications ranging from 0.8 μm prokaryotes to 100 μm plant cells. Single-cell dispensing is based on inkjet-type technology that generates free-flying microdroplets on demand that encapsulate cells to deposit them on variable substrates using a non-contact delivery process.
Dr. Zimmermann’s team chose the F.SIGHT single-cell dispenser given its advantages over methods such as fluorescence-associated cell sorting (FACS). While FACS is frequently used for single-cell isolation, that sorting process has a negative effect on the cloning efficiency of susceptible or partially damaged cells since it applies high shear forces and electrostatic charge to the cells.
The F.SIGHT is particularly suitable for cloning of engineered cells because, in addition to preserving their viability, it provides direct evidence of single-cell clonality and has both native and fluorescent sorting capabilities. In addition, the F.SIGHT can be coupled with single-cell analytical pipelines and requires far lower sample volumes to process than FACS, allowing researchers to isolate cells from limited samples. Finally, even when the fluorescence of the transfected cells is very limited, researchers can still identify the target population with the F.SIGHT, because it is able to read low signals.
Another innovative assay used in the workflow developed by Dr. Zimmermann’s team is emulsion coupling based on a digital method similar to droplet digital PCR (ddPCR), which detects the differences in partitioning of two antibodies labeled with target oligonucleotides in the presence of target proteins compared to their free and unbound distribution in ddPCR emulsion. Emulsion coupling is homogeneous, molecularly sensitive and quantitative. Plus, because of this assay’s two-antibody principle, it is highly specific and can be read by sequencing (e.g., next-generation sequencing) in a highly parallel manner.
A TECHNOLOGICAL SOLUTION DESIGNED TO OVERCOME THE CURRENT LIMITATION

- Genome-wide changes were detected using a surveyor assay
- Target gene transcript levels were monitored by RT-PCR
- Target protein expression by clones was measured by Emulsion coupling
All of these assays together allow the characterization of clones from thousands down to just a few hundred cells, facilitating low clonal expansion and high characterization throughput while still providing high-quality data about the clones. These assays facilitate success across cherry-picked clones. The workflow proposed by the team at the University of Freiburg is compatible with industrial requirements, highly automatable and can be easily transferred to other cell types and genetic targets.
Ultimately, we can say that the workflow developed by Dr. Zimmermann’s team can be adapted for other cell types and genetic targets and has great potential for many applications where monoclonal cells are required. Among the many benefits of this workflow is the lack of restrictions with single-cell dispensing technologies to handle different cell types. Plus, emulsion coupling reduces both the time and resources needed to potentially increase the content of characterization workflows, allowing for better clones with lower failure rates in the final stages of cell line development.
References:
Gross T, JeneyC, Halm D, et al. Characterization of CRISPR/Cas9 RANKL knockout mesenchymal stem cell clones based on single-cell printing technology and Emulsion Coupling assay as a low-cellularity workflow for single-cell cloning. PLoS ONE. 2020; 16(3): e0238330. DOI:1371/journal.pone.0238330.
EXPLORE THE BENEFITS OF F.SIGHT™
Single-cell dispensing made easy with fluorescent sorting
FEATURED WORKFLOW

Transfection

Transfection

Transfection