Induced pluripotent stem cells (iPSCs) are an incredibly powerful technology that continues to transform many facets of biomedicine. One particularly exciting area is the use of iPSC-derived cells as therapeutic agents. The development of allogeneic iPS cell-based therapies offers “off-the-shelf” therapies for regenerative medicine to treat conditions like cardiovascular disease, retinal degradation, and neurodegenerative disease, to name just a few (Fig. 1). Despite its therapeutic promise, allogeneic iPS cell‐based therapy development is a long and complex process with many challenges and potential pitfalls. This article will cover the core steps in developing these therapies, common barriers to success, and future directions in this exciting therapeutic field.
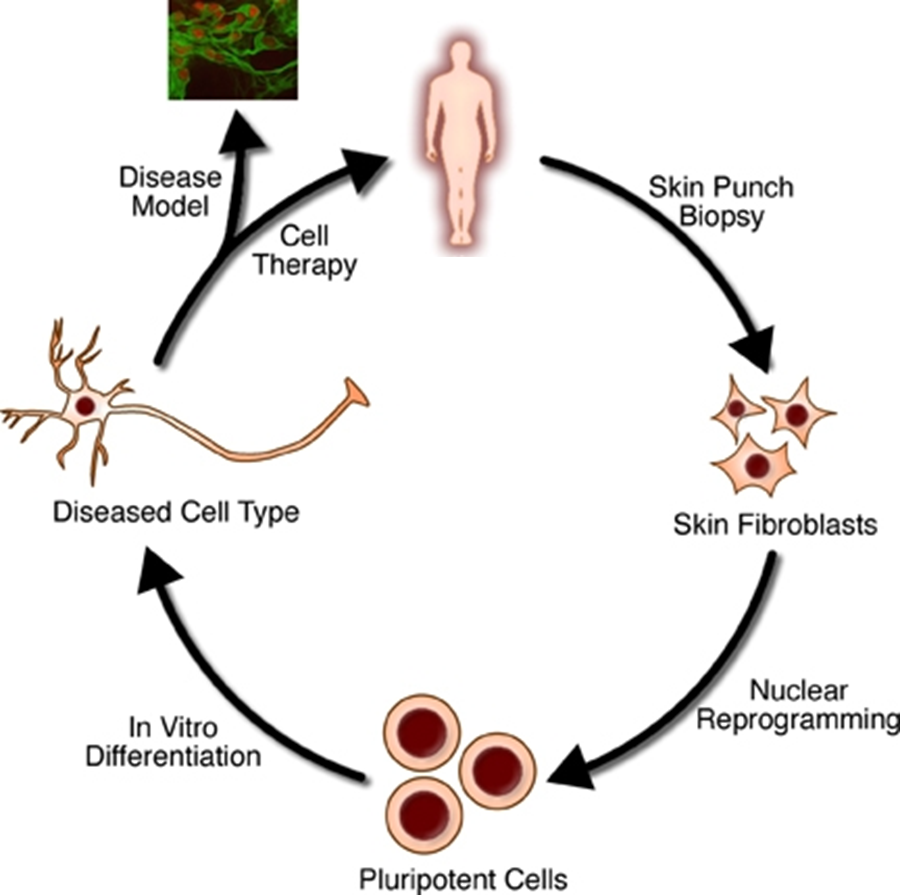
Figure 1. Somatic cells can be reprogrammed to a pluripotent state and then differentiated into different cell types for research and clinical applications.
Reprogramming and Modification
Allogeneic cell therapies differ from autologous therapies in that they are derived from an individual other than the patient and modified to enable their use in virtually any individual1. The process begins with the isolation of somatic cells from a consenting donor. These cells are reprogrammed from a differentiated state into a dedifferentiated, pluripotent state through exposure to different transcription factors. These factors, known as the Yamanaka factors, are named after Shinya Yamanaka, who pioneered their use in stem cell research in 2006.
Today, researchers incorporate additional factors beyond those used in the early iPSC techniques and several methods are available for delivering these factors to cells, including CRISPR- and RNA-based approaches. After reprogramming to become iPSCs, cells can be directed down different lineages to produce diverse cell types. Cells may also be genetically modified depending on the downstream application.
Selection
The next step is selecting high-quality clones with appropriate iPSC markers, and other desired genetic alterations. Monoclonal populations are required for cell-based therapies, and failure to demonstrate clonality can cause issues with regulatory bodies during drug development. The UP.SIGHT from CYTENA provides dual imaging and delicate single-cell dispensing that ensures a >99.99% probability of clonal derivation while preserving the viability of iPSCs, which are sensitive to shear stress (Fig. 2).

Figure 2: The UP.SIGHT ensures >97% single-cell dispensing efficiency and up to an 80% clonal recovery rate, helping streamline cell line development workflows.
Quality Control
Quality control (QC) is an essential part of any therapy development workflow. For iPSC-based therapies, QC is essential for ensuring pluripotency, identifying contaminants, and determining genomic stability. These steps are essential for ensuring the efficacy and safety of the final product and are required by regulatory bodies. QC is performed through a combination of pluripotency tests, genome sequencing, immunogenicity testing, and cell counting to ensure stable growth rates and viability. If iPSCs have been induced to differentiate, further functional tests may be performed, such as neuronal tests for synapse formation.
Scaling Up
Allogeneic iPSC‐based therapies possess the key advantage of being suitable for mass production. Autologous cell therapies are only suitable for a single patient and require smaller, more expensive, and less efficient workflows. On the other hand, allogeneic cell therapies can be scaled up and stored for use as “off-the-shelf” treatments for different diseases.
Storing
Cell banks are an essential part of allogeneic cell-based therapies. They allow therapies to be safely stored and accessed when needed. iPSCs are typically stored using cryopreservation at 196°C, and protocols usually involve intermediate cooling steps. Differentiated cells are also stored in cell banks. Regulatory bodies have strict guidelines for storing these cells, including further rounds of QC before thawed cells are shipped for clinical use and detailed record-keeping to ensure traceability.
Challenges
Generating allogeneic iPS cell‐based therapies is a complex process with many challenges at each step.
Loss of Pluripotency or Other Characteristics
iPSCs can lose their pluripotency over time. Loss of pluripotency can occur during the scaling-up phase in large culture volumes and can also occur after thawing. Robust QC measures are required to ensure cells are still fit for purpose at different stages of development.
The UP.SIGHT from CYTENA can achieve up to 80% clonal recovery for iPSCs without loss of pluripotency. We prepared an application note that showcases the UP.SIGHT’s strengths when handling iPSCs, along with optimization tips for effective iPSC effective culturing.. Read the full document here.
Immunogenicity of iPSC-derived Allogeneic Cell Therapies
Allogeneic cell therapies are derived from donors and face issues with immune rejection when implanted into patients. The HLA (human leukocyte antigen) gene is responsible for allowing cells to recognize self from non-self and is commonly edited to circumvent issues with immunogenicity2.
Costs
Though more efficient than workflows for developing autologous cell therapies, workflows for developing allogeneic iPSC-based therapies are relatively cost-intensive due to the need for extensive quality control and scalability challenges.
Future Directions
There are many exciting directions in which the development of allogeneic iPSC-based therapies is improving, including technologies that expand therapeutic applicability and streamline workflows.
Combination with Biomaterials and 3D Printing
iPSC-derived cell therapies are being combined with 3D biomaterial printing technologies to create novel tissue regeneration products. iPSC-derived organoids are set to play a huge role in drug development and testing, though they have not come to prominence yet.
Automation
Automated workflows are already helping to streamline cell line development, including iPSC-derived allogeneic cell therapies. Automation provides faster and more accurate liquid handling and assists in the selection of high-performing clones and the identification of clonal populations.
The C.STATION from CYTENA combines the UP.SIGHT with our other pioneering cell line development instruments, providing a fully automated end-to-end cell line development workflow.
Conclusion
Allogeneic iPS cell-based therapies offer immense promise for regenerative medicine, enabling scalable, “off-the-shelf” treatments for various diseases. However, their development is complex, involving reprogramming, selection, rigorous quality control, and overcoming challenges like immune rejection and loss of pluripotency. Despite these hurdles, advancements in automation, quality control, and novel combinations with 3D printing are streamlining the development process and pushing the boundaries of modern healthcare.
CYTENA is proud to support ambitious companies at the forefront of cell-based therapies. Book a demo to see firsthand how the UP.SIGHT can transform your cell line development workflow.
References
- Cerneckis J, Cai H, Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Sig Transduct Target Ther. 2024;9(1):112. doi:10.1038/s41392-024-01809-0
- Moy AB, Kamath A, Ternes S, Kamath J. The Challenges to Advancing Induced Pluripotent Stem Cell-Dependent Cell Replacement Therapy. Med Res Arch. 2023;11(11):4784. doi:10.18103/mra.v11i11.4784
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126(4):663-676. doi:10.1016/j.cell.2006.07.024
- Bailly A, Milhavet O, Lemaitre JM. RNA-Based Strategies for Cell Reprogramming toward Pluripotency. Pharmaceutics. 2022;14(2):317. doi:10.3390/pharmaceutics14020317
- Xue H, Wu J, Li S, Rao MS, Liu Y. Genetic Modification in Human Pluripotent Stem Cells by Homologous Recombination and CRISPR/Cas9 System. In: Turksen K, ed. Human Embryonic Stem Cell Protocols. Vol 1307. Methods in Molecular Biology. Springer New York; 2014:173-190. doi:10.1007/7651_2014_73
- Madrid M, Lakshmipathy U, Zhang X, et al. Considerations for the development of iPSC-derived cell therapies: a review of key challenges by the JSRM-ISCT iPSC Committee. Cytotherapy. Published online June 2024:S1465324924007308. doi:10.1016/j.jcyt.2024.05.022
- Jha BS, Farnoodian M, Bharti K. Regulatory considerations for developing a phase I investigational new drug application for autologous induced pluripotent stem cells-based therapy product. Stem Cells Transl Med. 2021;10(2):198-208. doi:10.1002/sctm.20-0242
- Yuan Y, Yang Y, Tian Y, et al. Efficient long-term cryopreservation of pluripotent stem cells at −80 °C. Sci Rep. 2016;6(1):34476. doi:10.1038/srep34476
- Chehelgerdi M, Behdarvand Dehkordi F, Chehelgerdi M, et al. Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy. Mol Cancer. 2023;22(1):189. doi:10.1186/s12943-023-01873-0
- Soman SS, Vijayavenkataraman S. Applications of 3D Bioprinted-Induced Pluripotent Stem Cells in Healthcare. Int J Bioprint. 2020;6(4):280. doi:10.18063/ijb.v6i4.280
- Doulgkeroglou MN, Di Nubila A, Niessing B, et al. Automation, Monitoring, and Standardization of Cell Product Manufacturing. Front Bioeng Biotechnol. 2020;8:811. doi:10.3389/fbioe.2020.00811




