- PRODUCTS
-
UP.SIGHT™ NEW
Optimized For Proof Of Monoclonality, Colony Tracking, Confluency, & Titer Measurement -
F.SIGHT™ 2.0
Optimized For Rapid Dispensing of Fluorescent Cells -
C.SIGHT™ 2.0
Optimized for Powerful Dispensing of Unlabeled Cells -
B.SIGHT™
Optimized For Rapid Microbial Single-Cell Isolation and Cultivation -
F.SIGHT™ OMICS
Optimized For Single-Cell-Omics -
F.SIGHT™
Optimized For Affordability And Flexibility -
C.SIGHT™
Optimized for Affordable Cell Line Development - Help Me Choose
-
UP.SIGHT™ NEW
- APPLICATIONS
- RESOURCES HUB
- COMPANY
- SHOP
Gene Therapy
Choosing the best clone for stable
viral vector production
viral vector production
Overview
Gene therapy is a new frontier for the treatment of several rare diseases and has, since the turn of the millennium, been adopted to treat diseases like Leukaemia, Parkinson’s and other specific cancers with precision. Gene therapy is often used to replace an affected disease-causing gene with a healthy copy of that gene. It can also be used to inactivate the respective faulty gene to stop disease progression.
There are many different products and procedures being developed to conduct gene therapy research more efficiently across a wide range of application areas. One of the most popular methods is Viral Vector Manufacturing. Due to the innate ability of viruses to introduce new genetic material to host cells, packaging healthy genetic material in viral vectors like adeno-associated viruses (AAVs)
or lentiviruses (LVs) has proven to be an effective strategy to incorporate healthy genes into
affected cells.

Sub-Applications
Cell line generation with
>99.99% probability of clonality
Generate clonally derived cell lines and decrease batch-to-batch variations for an improved viral vector manufacturing process.
Improve suspension cell culture and clonal expansion
Optimize cell culture and establish a high-throughput clonal expansion workflow to effectively select cell lines.
Reduce timelines
for clonal expansion
Save up to 13 weeks on your cell line development workflows and increase throughput from single-cell cloning to clonal cell culture and upscaling.
Research Workflows
Viral Vector Manufacturing
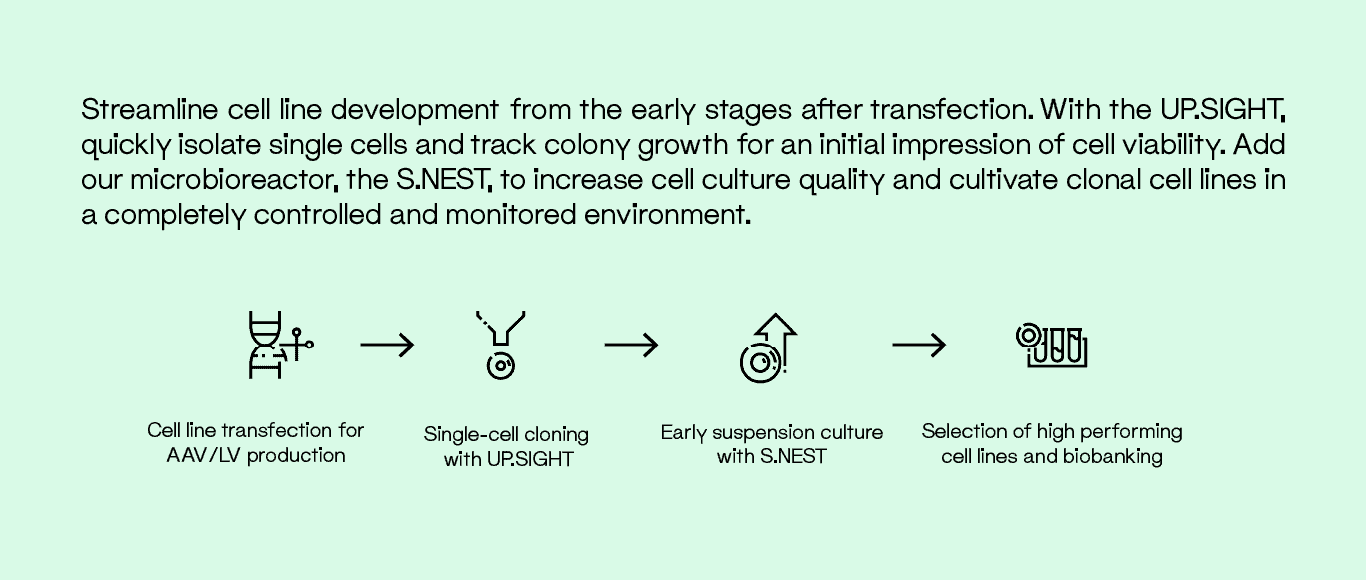
In order to produce AAVs or LVs that contain the transgene of interest, it is critical to develop stable viral vector producer cell lines from single cells and select the most efficient producer cell lines for further manufacturing. HEK293 and HEK293T host cell lines are routinely used to produce viral vectors at
high titers.
In order to comply with the FDA or EMA regulations for Viral Vector Manufacturing, our line of single-cell dispensers has been designed to optimize workflows while fulfilling such requirements. Both our F.SIGHT 2.0 and UP.SIGHT can sort single cells within minutes based on florescent signal, thus only dispensing cells of interest.
The UP.SIGHT platform, exclusively, has a dual imaging system that provides double assurance of monoclonality during single-cell isolation. Additionally, the UP.SIGHT is capable of imaging the whole plate for colony tracking, thus giving a full picture of colony development for subsequent selection of high-producing clones.
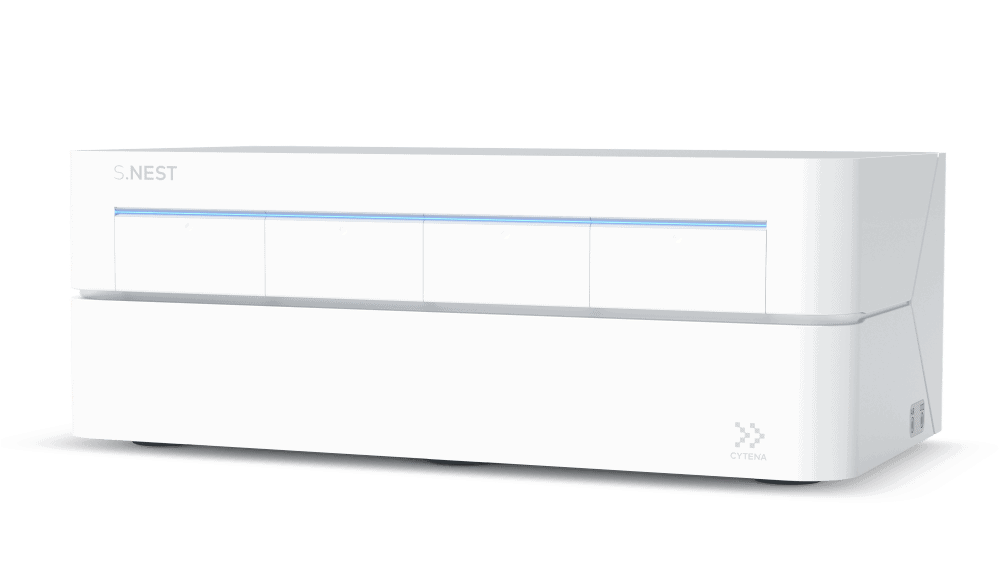
For ex vivo approaches in which cells are genetically modified during culture, the S.NEST is a high-throughput microbioreactor in which 24- or 96-well culture plates are continuously monitored to optimize and upscale clonal expansion. It offers continuous mixing for suspension cells in 96-well and 24-well plates by providing a higher diffusion rate of oxygen, which creates a more homogeneous
culture environment.
Featured Resources
Previous
Next